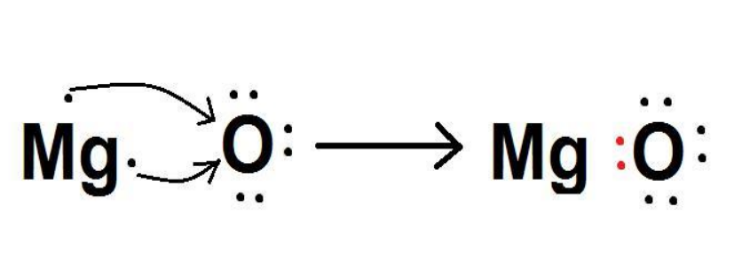Magnesium oxide is mainly prepared by the calcination of naturally occurring minerals, like magnesite (MgCO3).It is generally found in seawater, underground brine deposits, and metamorphic rocks.It is a white and odorless powdered compound. It is hygroscopic, i.e., it can absorb moisture from the air.It is highly basic as it reacts with water to form basic magnesium hydroxide. It has high melting and boiling point.MgO is a very useful compound. It is often used as an antacid because of its basic nature. Magnesia is used for making crucibles that are used in the chemical industry. It is also used in heating elements, fire-proofing materials, etc.So, is MgO ionic or covalent? MgO is an ionic compound. An ionic bond is formed by the transfer of electrons from a metal (Mg) to a non-metal (O). Mg loses two valence electrons to form Mg2+, which are gained by O to form O2-. Thus, an electrostatic interaction arises between two oppositely charged ions, and an ionic bond is formed.Let us study this in detail.
Why is MgO Ionic?
Magnesium is an alkaline earth metal that has 12 electrons and 12 protons in one atom of Mg.Mg tends to lose the two valence electrons to gain an octet configuration resembling Neon. (Nearest noble gas) In the Mg2+ ion, there are 10 electrons and 12 protons. As the number of protons is more than electrons, a net positive charge is present on magnesium ions.Electronic configuration of Mg: 1s22s22p63s2Electronic configuration of Mg2+: 1s22s22p6Mg → Mg2+ + 2e−Oxygen is a non-metal and has 8 protons and 8 electrons in an O-atom. It needs two electrons to complete the octet configuration resembling Neon. It takes 2 valence electrons of Mg.On gaining two electrons, there are 10 electrons and 8 protons. As the number of electrons is greater than protons, a net negative charge exists on the ion.Electronic configuration of O: 1s22s22p4Electronic configuration of O2-: 1s22s22p6O + 2e- → O2-Therefore, the two electrons which Mg lost were taken up by O. The two ions of opposite charges are formed in this process.These two ions exert an electrostatic force of attraction on each other, leading to bond formation between them. Thus, the bond formed between a metal and a non-metal by the transfer of electrons is called an ionic bond.
Factors Affecting Ionic Bonding of a Compound
The three factors that mostly affect the extent of ionic bonding are1. Ionisation Energy: We saw that Mg loses two valence electrons to form a divalent cation.All electrons are attracted to the nucleus by the electromagnetic force. To remove the electrons from the atom, some energy is required.The energy required to remove an electron from an isolated gaseous atom in the ground state is ionization energy.If ionization energy is more, it is difficult to lose electrons, and subsequent formation of cation is difficult and vice-versa.It is positive because energy is gained.2. Electron affinity: O-atom gains two electrons to form a divalent anion.Energy is released on gaining electrons because the addition of electrons causes stress on other electrons, and consequently energy is released.Electron affinity is the change in energy when an electron is added to a neutral atom in its gaseous phase to form a negative ion.The higher the magnitude of energy, the easier it is to gain electrons and form an anion. It is generally negative because energy is released.3. Lattice Energy: When cations and anions are brought close to each other, some energy is released.The amount of energy released when one mole of ionic solid is formed from the gaseous ionic constituents is lattice energy.It further depends on the magnitude of charges on cations and anions and the distance between ions. Lattice energy can be analyzed using the Born-Haber cycle.A high magnitude of lattice energy denotes the ease of formation of an ionic solid. Lattice energy is high when charges on atoms are high, and the distance between them is less.Low ionization energy, high electron affinity, and high lattice energy form the best combination for an ionic bond formation.
Percentage of Ionic Character in MgO
No bond is perfectly ionic or covalent. Every ionic bond has some covalent character, and every covalent bond has some ionic character.The percentage ionic character in a bond can be calculated by an empirical relation given by Linus Pauling.It correlates ionic character with the difference in electronegativity between two atoms.Where Δχ represents the difference in electronegativity.In MgO, electronegativities of Mg and O are 1.2 and 3.5, respectively. Δχ is 2.3. Percentage ionic character= (1- e-((2.3/2)^2)) * 100= 73.35%
Fajan’s Rule
Covalent character in ionic compounds and vice-versa can be compared using postulates of Fajan’s rule.According to Fajan’s rule, a compound with a small cation, large anion, and high cation charge is considered more covalent.If the size and charge come out to be the same, then it is said that transition metals have a greater covalent character than s block cations.A small cation has a higher charge density and can easily polarize the anion. In a larger anion, the valence electrons are far from the nucleus, and the effective nuclear charge is less, due to which it can be polarized easily.Higher polarizability of anion and higher polarizing power of cation favor covalent character.Example] Both CaO and MgO are ionic compounds, but CaO is more ionic than MgO.This can be attributed to the larger size of Ca2+ and hence less polarizing power. Even the percentage ionic character of CaO is ~79%.Check out the article on the ionic character of CaO.
Comparison Between Ionic and Covalent Bonding
Atoms and molecules are kept together in a compound by the attractive force, which stabilizes them by decreasing the system’s energy.This is called chemical bonding. Primarily, bonding is of two types.
General Properties of an Ionic Compound
• Physical properties– Cations and anions are attracted towards each other due to electrostatic force.This electrostatic force of attraction makes ionic compounds strong, and they are not broken easily. They are brittle as they break into small pieces by applying pressure.• Melting and Boiling Points: A large amount of energy is required to break the strong ionic bonds between atoms.Thus, the melting point and boiling point for ionic compounds are very high.• Solubility– Ionic compounds are generally polar. Following the thumb rule of “like dissolves like” ionic compounds are soluble in polar solvents.As expected, they are mostly insoluble in non-polar solvents.• Electric Conductivity– Ions can conduct electricity, but ions cannot move in an ionic solid.Thus, solid ionic compounds do not conduct electricity. If they are allowed to move freely, they can conduct electricity, which is possible either in the molten or aqueous form of an ionic solid.• Geometry and Shape– We cannot describe the geometry and shape of the formula unit of ionic compounds because the ionic bonds are non-directional.Ions are attracted to each other by the electrostatic force of attraction which is non-directional in nature.• Structure of lattice– An ionic compound can be regarded as a giant structure of ions. The ions have a regular repeating structure called an ionic lattice.The structure depends on the comparative size of cations and anions. For instance, Ionic solid MgO exists in a cubic type crystal system.
Conclusion
MgO is an ionic compound formed by the complete transfer of 2 electrons from the valence shell of Magnesium metal to the outermost shell of the O- atom.• An ionic bond is formed due to electrostatic forces of attraction between magnesium cation and oxygen anion.• Percentage ionic character in MgO- 73.35%• MgO is a very useful compound that finds its applications in various industries.