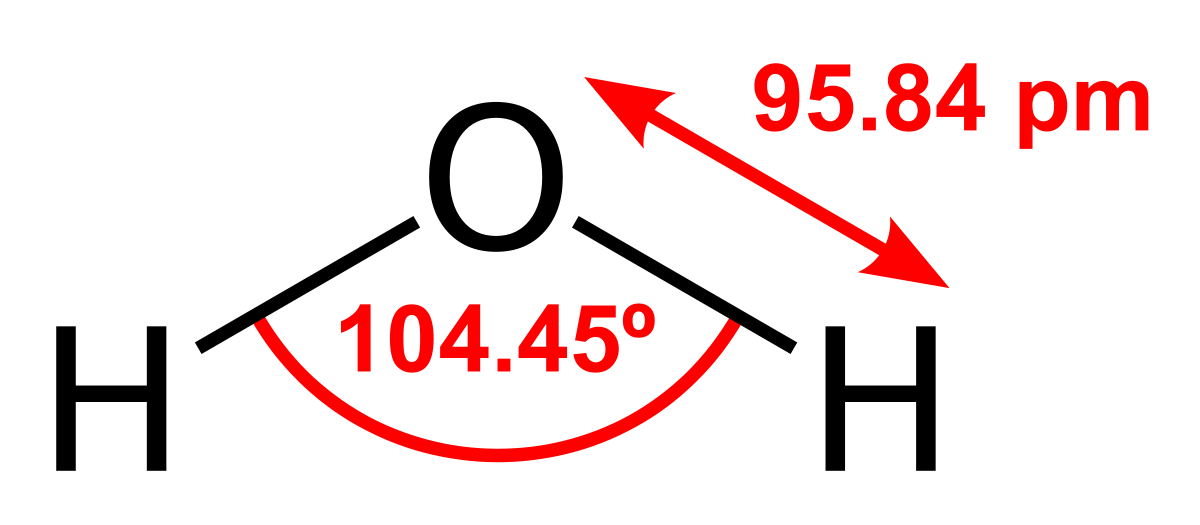
The molar mass of water is 18 g/mole. The boiling and melting point of water are 373K and 273K, respectively.Water obtained through sea, lakes, taps, or even purifiers is not pure water. Water contains various impurities in the form of essential minerals and contaminants. We drink water after the removal of contaminants.When contaminants and essential minerals are removed from the water, we get distilled water.In this article, we will understand the meaning and properties of a pure substance and a mixture. We will also classify distilled water and tap water under these categories.So, is distilled water a pure substance? Distilled water is a pure substance as only water molecules are present in a given sample of distilled water. It is a compound made up of hydrogen and oxygen atoms only and has definite properties and composition throughout the sample.Let us check it in more detail. Keep reading.
What is a Pure Substance?
The matter is anything that has mass and occupies space. Matter can be classified into pure substances and mixtures based on chemical composition.Pure Substances are those which have uniform composition and definite properties throughout. They are made of only one type of atom or molecule.Pure substances are further classified as elements and compounds.Elements are often regarded as the basic form of matter that cannot be broken down. Only one type of atom is present in an element. All atoms have the same number of protons.Compounds are made up of atoms of two or more elements and are combined chemically in a fixed proportion. A compound has only one type of molecule.For example, Copper is a pure substance because a copper piece contains only copper atoms. It belongs to the class of elements.Sodium chloride is a pure substance because a pinch of salt contains NaCl crystals only. Na and Cl combine together in a fixed ratio. It belongs to the class of compounds.
Why is Distilled Water a Pure Substance?
A sample of distilled water contains only water molecules.All impurities like dissolved salts, colloidal particles, and important minerals like Fe, Zn, Cu, Ca, P, Mg, etc., are removed by chemical and physical treatment from ordinary water available.These impurities are present in varying amounts in water at different places and affect water’s physical and chemical properties.On their removal, only water molecules are left.In water, oxygen and hydrogen atoms combine in a 1:2 ratio.Properties like density, melting point, boiling point, etc., are constant for distilled water.Distilled water is a compound because it comprises only one type of molecule. It is a pure substance because of its definite composition and properties.
What is a Mixture?
A mixture is made up of pure substances. A mixture is formed when two or more pure substances are mixed together without reacting.A mixture can be homogeneous or heterogeneous.The mixtures which have a uniform composition throughout, and we cannot tell that they are mixtures just by looking is called homogeneous mixtures. True solutions are homogeneous mixtures.For example, we cannot distinguish between salt and water when we mix salt in water and stir the solution vigorously. Single-phase is seen.Those mixtures which do not have a uniform composition throughout, and we can tell that they are mixtures just by having a look are heterogeneous mixtures.For example, we can distinguish sand from water when we mix sand in water and stir vigorously. There are two distinct phases. Sand settles at the bottom of the container, and its composition is not uniform throughout.Suspensions are heterogeneous mixtures in which solid particles are suspended in the bulk of the solution. We can see the solid particles from our naked eyes. Sand in water is a suspension.Colloids are heterogeneous mixtures in which solid particles are smaller than those in suspension. We cannot always distinguish a colloid and a true solution by our naked eyes. Milk is an example of a colloid.
Is Distilled Water a Mixture? Why or Why not?
Distilled water is formed by removing every foreign chemical substance from water. Only water molecules are present.It is not a mixture because it comprises only one type of molecule. Water molecule is made up of two pure substances (H and O) bonded together chemically.For a mixture, the pure substances should just mix without forming chemical bonds, and this does not happen in distilled water.H2O Lewis Structure, Geometry, Hybridization
Pure Substance v/s Mixture
The matter is classified into pure substances and mixtures. Here is a comparison between these two categories. – cannot be broken down further.-composition is uniform and fixed in a given piece of zinc.-impurities are absent in a pure zinc piece.-sand settles at the bottom, so the composition is not uniform.– sand can be separated from water by physical methods.– physical and chemical properties are not definite as the composition is not fixed.
Why is Tap Water a Mixture But Distilled Water is not?
Tap water is not purified. It contains all the impurities, minerals, air, chlorine, etc. The exact composition of tap water depends on the region. It is generally a homogeneous mixture.Tap water is a mixture because-• It contains different molecules (impurities and water), while distilled water contains only water molecules. Tap water conducts electricity because of dissolved salts present, unlike distilled water.• The composition and properties are not definite. It depends on the region. The composition and properties of distilled water are the same everywhere.• The impurities in tap water can be separated by physical processes, while no such separation can occur in distilled water.
Properties of a Pure Substance
A pure substance is of utmost importance. We have discussed a lot about pure substances in the above sections.Some of the basic properties of a pure substance are-– A pure substance is made up of only one type of species.– The composition is fixed and uniform throughout.– Pure substances are mostly homogeneous.– We can separate a pure substance into its constituents by a chemical process but not by physical process.– The properties of a pure substance are well defined– Pure substance forms predicted products after undergoing a chemical reaction.
How is Distilled Water obtained from Tap Water?
When we prepare distilled water from tap water, we remove the contaminants and minerals to obtain only water molecules. It is done by the process of distillation.Distillation is used to separate the components of a liquid mixture. The components can be separated only if the difference between their boiling points is more than 25K. If it is less than 25K, fractional distillation would be needed.In this process, we have to boil the water to form vapors. The vapors are collected and condensed in another container. This process works because only water molecules form vapor at the boiling point of water, and other impurities are left behind.Tap water is taken in a round-bottomed flask. The flask is heated till all water molecules are converted to vapor.The temperature remains constant throughout the process. The vapors are directed from the distilling flask to the condenser, where it is cooled to form liquid water. This liquid water then moves to the receiving flask.Activated carbon charcoal can be used to filter out any impurities that might have passed in the distillate.There might be some impurities that have a lower boiling point than water. We have to keep a check on the temperature. Any distillate obtained below 373 K has to be discarded.Sometimes one final step of filtration in a filtration media is needed.It is a slow process, and much fuel is required.
Conclusion
Pure substances are made up of only one type of atom or molecule and have a fixed and uniform composition.Distilled water is a pure substance because it has only one type of molecule: water molecule. The composition and properties of distilled water are uniform throughout.A mixture is made of two or more pure substances that do not form a chemical bond, and the composition is not fixed.Tap water is a mixture because it contains various contaminants, minerals, and air which are removed while forming distilled water. These impurities are of varying compositions.Happy learning!!Related article; Does distilled water conduct electricity