It belongs to the class of chlorofluorocarbon (CFC) compounds. It is an alkane derivative. Alkanes are saturated hydrocarbons composed of carbon and hydrogen only. In CFCs, all hydrogens are replaced.In this article, we will discuss the polarity of CF2Cl2 and the factors affecting the same.So, is CF2Cl2 polar or non-polar? CF2Cl2 is a polar compound due to its asymmetric shape. The C-F and C-Cl bonds are polar due to the difference in electronegativities (C is the least electronegative). The dipole moment vectors of bonds inside molecules do not cancel out each other because of asymmetry, and the compound as a whole is polar.The mass of CF2Cl2 is 120.91 grams/mole. The melting and boiling points are -158°C and – 30°C, respectively. It is transported as a compressed liquefied gas.It is soluble in organic solvents like alcohol, ether, etc. It is a non-flammable substance. It is chemically inert because of the high stability of C-F bonds.On a laboratory scale, freon 12 is synthesized using the following reaction.CCl4 + HF + SbF3Cl2 (catalyst) —–> CFCl3 + CF2Cl2 + HClR-12 is commonly used in cleaning products, refrigerants, degreasers. It is also used in submarines and aircraft as a fire retardant.The point group of the molecule is C2v. This implies that rotation of the molecule by 180° gives an equivalent structure, and there are 2 planes of symmetry in the molecule.F and Cl have a -1 oxidation state, while C has a +4 oxidation state in this compound. Montreal protocol bans the production of CF2Cl2 in many countries as it has a negative impact on the ozone layer. It has been classified as a greenhouse gas.Let get started studying it in more detail.
Why is CF2Cl2 Polar?
In CF2Cl2, there are two types of bonds, C-Cl and C-F.Both Cl and F are more electronegative than C. The electronegativity of C, Cl, and F are 2.55,3.16, and 3.98, respectively. This difference leads to polar bonds. Dipole moment vectors in both cases are directed from C to Cl/F. There is a partial positive charge on C and a partial negative charge on F and Cl. Polar bonds do not guarantee a polar molecule.It is a tetrahedral compound. It should have been symmetrical, but due to different side atoms, the compound is not symmetrical.It would have been symmetrical if all the side atoms were the same.The magnitude of dipole moment is different for the two types of bonds due to which net dipole moment is non-zero, and the compound is polar.
Lewis Structure of CF2Cl2
Lewis structure depicts the most basic arrangement of atoms in a molecule. The basis for Lewis Structure is the octet rule.We choose the least electronegative atom as the central atom.There are 4 valence electrons on C and 7 on Cl and F.These valence electrons are arranged around atoms such that the octet of all the atoms is complete.C shares one valence electron with each side atom, and all the side atoms also do the same.
Why is CF2Cl2 Tetrahedral?
The shape of any covalent compound can be determined by VSEPR theory.According to VSEPR theory, electrons in the valence shell of atoms in a molecule repel each other, and they arrange themselves in a way to make this repulsion minimum.This stable arrangement of the electrons is called geometry.
Steps to find the Geometry of CF2Cl2
Step 1. Count the number of valence electrons on the central atom, i.e., C in this case. The electronic configuration of C is 1s2 2s2 2p2.The valence shell has 4 electrons.Step 2. For each side atom, we add 1 contribution to the central atom.There are 4 side atoms in CF2Cl2, so 4 contributions to the central atom and total become 8.Step 3. Dividing 8 by 2, we get that there are 4 pairs of electrons.Step 4. This table predicts shape and geometry using the total number of electron pairs and the general formula.Thus the shape of CF2Cl2 is tetrahedral.
What is Polarity?
One of the essential physical properties of a compound is its polarity.When the shared electrons are not shared equally between two atoms, the atoms develop partial charges.The more electronegative atom tends to attract the shared pair of electrons towards itself and develops a partial negative charge.In contrast, the less electronegative attracts the shared pair of electrons to a lesser extent and develops a partial positive charge.This separation of opposite charges results in a dipole, and the bonds are said to be polar.
Difference Between Polar and Non-polar Compounds
Factors Affecting Polarity of CF2Cl2
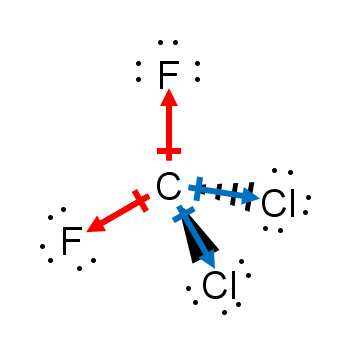
The development of unequal partial charge in a molecule can be induced due to many reasons. Major reasons are1. Dipole moment – It is a vector quantity and is the product of the magnitude of charges and the distance between them.It is often referred to as the indicator of polarity. There is a dipole moment only when the charges separate.The SI unit of dipole moment is Debye. It can be present in ionic and covalent compounds.The formula for calculation of Dipole moment isµ (Cm) = Q (C) * r (m)where µ is dipole moment,Q is the magnitude of charges, and r is the separation distance between two charges.Below is the image showing the direction of dipoles inside the CF2Cl2 molecule.2. The difference in electronegativity – Generally, no two elements have the same electronegativity.There is always some difference in electronegativity between two atoms, no matter how small.This difference in electronegativity allows one of the atoms to develop a negative charge by attracting the shared pair of electrons towards itself.Similarly, the other atom develops a partial positive charge to maintain the net charge on a molecule. Thus, a dipole is created due to different electronegativities.3 Geometry and Symmetry – There is no effect of geometry if the bonds are non-polar or diatomic. Diatomic bonds are always linear, so we need not consider geometry.For polar bonds, geometry is a major deciding factor.If the geometry of a compound is symmetrical like linear and all the side atoms are the same, all the dipole moment vectors point in opposite directions and cancel each other out.This makes the compound non-polar. If the geometry is not symmetrical like bent, dipole moment vectors do not cancel out.The asymmetry can also be introduced by different side atoms; as in the case of CF2Cl2, the magnitude of dipole moment would differ.4 Charge Separation – The distance between the two partial charges also affects the magnitude of polarity.
Why is CF2Cl2 Covalent?
In covalent compounds, bond formation takes place by sharing of electrons between atoms. For facilitating sharing, the electronegativity difference should be less than 1.5 between the atoms.If it is more, sharing won’t be possible as one atom would attract the shared pair more towards itself.The electronegativity difference between C and F is 1.43 (3.98-2.55), while between C and Cl, it is 0.61 (3.16-2.55).A covalent bond is formed between non-metals and C, Cl, and F; all are non-metals. No compound is entirely covalent or ionic.Every covalent compound has some ionic character in it, and every ionic compound has a covalent character.The relative covalent character can be compared using Fajan’s rule.Following factors favor covalent character in a compound-• The small size of the cation• The large size of anion• More charge on the cation
Why is CF2Cl2 Banned?
The harmful radiations coming from the sun are absorbed in the stratosphere region of the atmosphere by the ozone layer.When CF2Cl2 reaches this zone, UV rays break this down to release Cl-atoms which break down ozone to molecular oxygen and free O-atoms.Thus, the ozone layer is depleted.Montreal protocol bans the substances that deplete the ozone layer. The production of CF2Cl2 is banned in some countries under this protocol.
Uses of CF2Cl2
- The refrigerant in refrigerators and air conditioners.2. Fire retardant in submarines and aircraft.3. Aerosol spray propellants like body spray, hair spray, etc.4. Household cleaning products.5. Construction and building materials.
Conclusion
CF2Cl2 is a polar compound.All the four bonds are polar due to the difference in electronegativities of C and F/Cl. Since F is more electronegative than Cl, C-F bonds are more polar than C-Cl bonds.Due to the tetrahedral shape, all the bond moment vectors point in a different direction but do not cancel out because the magnitude of the dipole moment is not the same.The net dipole moment of the compound is non zero which makes it polar.It is a covalent compound as the difference in electronegativity of elements in the molecule is less than 1.5.Happy Learning!