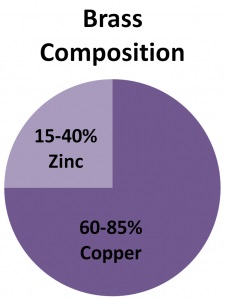
The percentage of the two main metals, viz. copper and zinc, may be altered to vary the properties resulting in the formation of different varieties of brass.Historically, brass has been used since Neolithic times. Brass is a kind of substitutional alloy where the atoms of the two metals may swap their positions inside the crystal structure.So, is Brass a mixture? Yes, Brass is a mixture made up of copper and zinc. It is a mixture because the two elements only combine physically to form brass and there is no chemical bonding taking place. Usually, brass is formed with 65% copper and 35% zinc. However, the percentage may vary in different samples of brass which is also a property of mixtures. As we already know there are three types of substances, viz. elements, compounds, and mixtures.Elements are made up of only one type of atom, for example, copper, zinc, etc.Compounds are made of one or more types of atoms combined together in a definite ratio, for example, water, glucose, etc.In a compound, the combining atoms chemically bond with each other and require extensive processes for separation.Finally, there are mixtures in which the two or more types of atoms or molecules come together in space and merge physically with each other.The properties of the mixture so formed may differ from any of the combining atoms or molecules even when they do not bond chemically with each other. Also, the percentage of the constituent atoms or molecules may vary from one sample to another. As discussed earlier copper and zinc physically combine in different proportions for the formation of brass. Therefore, brass is a mixture.
Is Brass a Homogeneous Mixture?
Homogeneous mixtures are those in which the composition of the combining atoms or molecules remains uniform throughout the mixture. Brass, which is an alloy of zinc and copper, is also categorized as a homogenous mixture because the percentage of the combining atoms remains uniform, and also the physical properties are in line throughout the sample i.e. the constituent atoms cannot be distinguished from each other.The metals are liquefied at high temperatures and then dissolved with each other for the formation of alloys due to which their composition remains the same throughout the sample.Therefore, all the alloys are placed in the category of homogeneous mixtures.Few metals however do not blend with each other even in the liquid state and hence, cannot be used in the formation of alloys, for example, gold and lead.
What is a Homogeneous and Heterogeneous Mixture?
The mixtures are divided into two categories based on their properties. They are homogeneous and heterogeneous mixtures.Homogeneous mixtures, as the term itself, indicates ‘homo’ means same, are formed when the constituent atoms or molecules are uniformly distributed throughout the mixture.Further, the components are so closely involved that they cannot be differentiated through naked eyes. These can be easily confused with pure substances but are differentiated based on the proportion of the components.The proportions or percentage of different components in a homogeneous mixture may vary from one sample to another.The alloys are an excellent example of homogeneous mixtures in which the two or more atoms combine to form a third substance the properties of which vary from its constituent elements.However, the individual components still retain their original properties.For example, brass has different properties than zinc and copper.‘Hetero’ means different. Therefore, heterogeneous mixtures are those in which there is no uniformity of the combining atoms or molecules.The percentage of the components may vary within a single sample and they can be easily distinguished from each other by mere visual observation and therefore, can be easily separated from each other.For example, in soil different components are mixed together which can be easily separated from each other, Colloids and suspension are also an example of heterogeneous mixtures.
Why is Brass not a Pure Substance?
The matter is anything that has mass and occupies space. It can be further divided into two types: pure substances and mixtures.Pure substances are formed of only one type of atoms or molecules combined in defined proportion i.e. they have a constant structure.Also, they have definite properties viz. fixed melting and boiling point, etc.Brass is not a pure substance as it is formed by the combination of two different types of atoms viz. zinc and copper.Also, the proportion of different components differs from one sample to another, therefore, brass does not have a defined structure.The properties also vary from sample to sample owing to change in the percentage of constituent metals.
Is Brass an Alloy?
Yes, it is an alloy of zinc and copper.The two metals combine to form brass along with a few other substances which are meant to enhance certain properties.The usual percentage of combination is 65% copper and 35% zinc. However, the proportions differ which also result in varying properties.Actually, the percentage is also varied on purpose to form different varieties of brass that are intended for different uses.
What is an Alloy?
An alloy is defined as an admixture of two or more metals or different elements combined with one metal. The properties of an alloy are completely different from any of the individual constituent metal or this can be rightly said that alloys are meant to form a better version of metals with improved properties likes increased strength, corrosion resistance, etc. For example, brass is more malleable than any of its constituent metals.Alloys behave differently from metals but retain the important properties of metals like ductility, electrical conductivity, luster, etc.Alloys can further be classified as substitutional or interstitial alloys and homogeneous or heterogeneous alloys.Brass is a form of substitutional alloy in which the atoms are able to replace each other in the crystal structure.Alloys also occur naturally, for example, Electrum is an alloy of gold and silver, meteorites also consist of some natural alloys.The first alloy invented by humans was brass, formed by combining copper and tin.They are prepared by heating the base metal (larger percentage) to liquefy it and then dissolving the other constituents in it.
What is Brass Made of?
The basic components of brass are zinc and copper with traces of other components like arsenic, aluminum, phosphorous, manganese, and silicon.However, the proportion of these two metals varies for different varieties of brass.The percentage composition and important properties are listed below:• Alpha Brass: They consist of 65% copper and 35% zinc. Due to the high percentage of copper, they have a gold-like appearance. For example, red brass.• Alpha-beta Brass: They consist of 55-65% copper and 35-45% zinc. They are also known as duplex brasses and are brighter in appearance.• Beta Brass: They consist of 50-55% copper and 45-50% zinc. They are stronger and harder and are meant for use in hot environments.• Gamma Brass: They consist of 33-39% copper and 61-67% zinc. Also consists of gold (30-50%) or Au (40%).• White Brass: They consist of <50% and >50%. Due to the high percentage of zinc, they are called zinc casting alloys with copper additives.
Properties
• In appearance, brass ranges from golden to silvery white depending upon the percentage of copper and zinc.• The melting point of brass is 900 – 940°C.• It has a density of 8.4 – 8.73 g/cm3.• It is tarnish-resistant and has low friction.• It is more malleable than zinc or copper or bronze.• It is a good conductor of heat.• It has acoustic properties.• It is resistant to corrosion.• It is not ferromagnetic due to which it can be easily recycled.• It also has anti-microbial properties.• It is prone to stress corrosion cracking when exposed to ammonia.
Uses
• It is used in making musical instruments owing to its acoustic properties.• It prevents biofouling due to its bactericidal properties.• It is used in making locks, gears, valves, brackets, base plates, etc.• It is used in making braces for teeth.• Due to its gold-like appearance, it is also used in making decorative items.• It is used in making plumbings like pipes, tubes, etc.• It is used in making tools and fittings used in explosives.• Cartridge cases are also made of brass.• It is used in making weather strippings.• It is used in making radiators and screws.• In ancient times brass was used for making jewelry, armors, vessels, etc.
Conclusion
Brass is a mixture of copper and zinc. It is a mixture because the two elements only combine physically to form brass and there is no chemical bonding taking place.Brass is a homogenous mixture because the percentage of the combining atoms remains uniform and also the physical properties are in line throughout the sample.Homogeneous mixtures are formed when the constituent atoms or molecules are uniformly distributed throughout the mixture while heterogeneous mixtures are those in which there is no uniformity of the combining atoms or molecules.Brass is not a pure substance as it is formed by the combination of two different types of atoms and the proportion of different components differs from one sample to another.Brass is an alloy of zinc and copper, the usual percentage of combination is 65% copper and 35% zinc. An alloy is defined as an admixture of two or more metals or different elements combined with one metal.Happy learning!!