Every cell of the body contains its own DNA which is unique to every individual. The different individuals, even when they belong to the same species do not have identical DNA except for the congenital twins that are born with the same DNA.Due to its uniqueness DNA also serves as a source of identification. Most DNA is located inside the nucleus of a cell. However, another type of DNA called mitochondrial DNA which is maternally inherited is located in the mitochondria of the cell.An interesting question regarding bonds inside the DNA molecule is whether DNA consists of hydrogens bonds in it or not.So, does DNA have hydrogen bonds? Yes, the two strands of the DNA molecules are joined through hydrogen bonds. The purines always bond with pyrimidines, however, the number of bonds differs between the two types of base pairing. The adenine molecule forms a double bond with thymine while guanine forms a triple bond with cytosine. Therefore, multiple hydrogen bonds exist between the complementary nucleotides located on opposite strands of DNA.Let us study the bondings in DNA in detail.
How are DNA molecules Bonded?
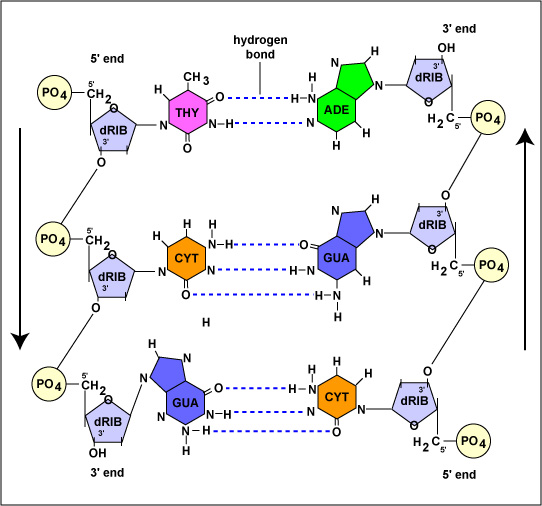
DNA is a double-stranded structure that serves as a carrier of biological information for most organisms.The two strands of DNA are composed of nucleotides and each nucleotide is a combination of three molecules viz. a deoxyribose sugar (pentose), a nitrogenous base, and a phosphate group.A large number of nucleotides bond together to form one strand of DNA. Human genome consists of about 3,20,00,000,00 nucleotides.Looking at the structure of a DNA strand it is observed that the phosphate group is attached to the hydroxyl group (5’) of one sugar molecule, forming an ester bond on its one side, and the 3’ carbon atom of the adjacent sugar molecule.As two ester bonds are formed through a phosphate link, this bond is termed phosphodiester linkage. These sugar-phosphate bonds form the backbone of DNA molecules.The structure of nucleotides differs owing to the presence of four different types of nitrogenous bases viz. adenine, cytosine, guanine, and thymine.These bases bond with their complementary base present on the opposite strand of DNA through hydrogen bonds. As per Chargaff’s rule adenine always bonds with thymine through a double bond while guanine always bonds with cytosine through a triple bond.These bonds are responsible for binding the two strands of DNA molecule together thus making it stable.Amongst these adenine and guanine are double ring structures and are called purines while thymine and cytosine are single ring structures and are classified as pyrimidines. Essentially, purines bond with their opposite pyrimidines and vice versa.The two strands of DNA are coiled in a right-handed fashion and are anti-parallel to each other i.e. one strand runs from 3’-5’ end while the other strand runs from 5’-3’ end.This coiling and twisting of DNA make it rather more stable and less susceptible to changes in the outside environment.
Importance of Hydrogen Bonds in DNA
As we have already seen in the previous section the nitrogenous bases are bonded together through hydrogen bonds and these hydrogen bonds are responsible for holding the two strands of DNA together.Hydrogen bonding actually plays a very crucial role inside a DNA molecule. The foremost reason is stability. As DNA works as the hereditary material it is very important that it should be a stable structure.If the two strands are bonded through weak linkages such as Vander Wal forces it might lose some critical or vital information while passing from one generation to another.Also, we know that DNA molecules undergo a replication process through which multiple copies of DNA are formed. These are required for storage and transfer of information, repairing process, etc.For replication of DNA first, the two strands are separated from each other and thereafter, complementary base pairs are added to form another copy of DNA.This is an energy-consuming process and the amount of energy required to separate these strands is directly proportional to the strength of the bonds.If the bonding between the two strands is really strong like in the case of covalent bonds, it would require the wastage of ample amount of energy which may otherwise be used for some productive purpose.Therefore, it is clear that the bonding between two strands should be strong enough to provide stability but weak enough to be energy efficient for the replication process.Here, hydrogen bonding serves the purpose as they are neither as strong as covalent bonds nor as weak as Vander Wal forces, the strength of these bonds is just correct for a DNA molecule to be stable as well as replication ready.Other than this hydrogen bonds are also help proteins and enzymes in the recognition of DNA molecules which is required for various biological purposes.As hydrogen bonds are formed between complementary base pairs one strand of a DNA molecule is an exact reverse copy of the other strand.Therefore, it also helps keep the backup copy in case one strand gets damaged due to any reason.
Structure of DNA
The structure of a DNA molecule is a double helix. This was discovered by two scientists James Watson and Francis Crick and therefore, is popularly known as Watson and Crick model.Each strand of DNA is a polynucleotide which means that it is formed of repeated monomeric units of nucleotides.As discussed earlier each nucleotide is formed of a molecule of deoxyribose sugar, one phosphate group, and a nitrogenous base.The nitrogenous bases are further of four types viz. adenine, guanine, thymine, and cytosine.The nitrogenous base is always attached at the 1’ carbon of the sugar and by counting carbons in the anti-clockwise direction we can see that the phosphate group is attached to the 3’ carbon of one deoxyribose sugar and 5’ carbon of the neighboring sugar molecule.The sugar molecule in DNA is referred to as deoxyribose as it does not contain the hydroxyl group (–OH) on the 2’ carbon unlike ribose sugar and therefore, the nucleotides of DNA are called deoxyribonucleotides.In a DNA strand nucleotides attach to each other through phosphodiester linkage in which the phosphate group of one nucleotide, present on its 5’ carbon atom of its sugar molecule, attaches to the oxygen atom present on the 3’ carbon of the sugar molecule of the next nucleotide.The carbon number mentioned above are used in deriving the direction of the DNA strands which is considered to be from 5’ to 3’.However, the two strands of a DNA molecule run anti-parallel to each other which means if we try to find the direction of both strands looking at one end of the DNA molecule one strand runs from 5’ to 3’ while the other strand runs from 3’ to 5’.The two nucleotides on one strand of DNA are bonded through a covalent bond but the nucleotides present on opposite strands are linked through hydrogen bonding.Each nitrogenous base forms multiple hydrogen bonds with its complementary base present on the opposite strand. Each of these units bound together through hydrogen bonds is known as a base pair.As discussed earlier adenine always bonds with thymine (double bond) while guanine always bonds with cytosine (triple bond).Thymine and cytosine are pyrimidines and have a single ring structure while guanine and adenine are purines and have a characteristic double-ring structure.The reason for this specific bonding is the geometry of these bases. Adenine and thymine have similar geometry which allows symmetry in the structure of DNA molecules and are thus able to make stable hydrogen bonds.The same is the case of cytosine and guanine.This actually depends on the distance between the base pairs and also the angle at which the base pairs are attached to the backbone. Other base pairs being different in geometry disturb the helical structure if bonded.The structure of the DNA molecule is uniform. Each turn of the helix consists of 10 base pairs.Single Strand of DNA is as belowDouble-stranded DNA with complementary base pairing
How many Hydrogen Bonds are there in DNA?
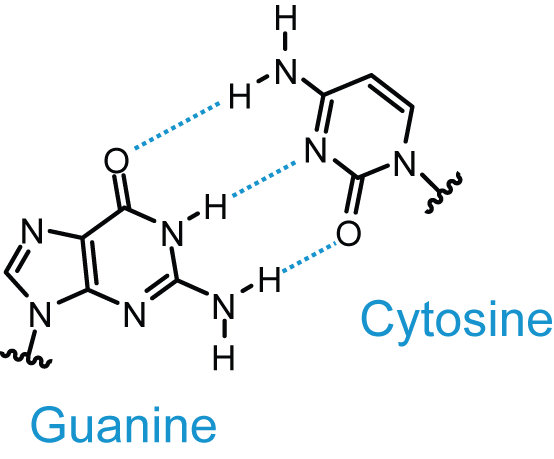
The number of hydrogen bonds in a DNA molecule depends upon the nitrogenous bases present in the molecule.As discussed earlier adenine and thymine are connected through two hydrogen bonds amongst which one bond is formed between a hydrogen atom of the amino group located at the sixth carbon of adenine and the oxygen atom of the keto group located at the fourth carbon of thymine.The other bond is between the nitrogen atom at the first position of adenine and the hydrogen atom connected to the third nitrogen of thymine.In the case of guanine and cytosine, three hydrogen bonds are formed between the oxygen atom of the keto group located at the second carbon of cytosine and a hydrogen atom of the amino group located at the second carbon of guanine, between the third nitrogen atom of cytosine and the hydrogen atom attached to first nitrogen of guanine, and the hydrogen atom of the amino group located at fourth carbon of cytosine and oxygen atom of keto group located at sixth carbon of guanine, respectively.Therefore, the number of hydrogen bonds in a DNA molecule can be given by the following formula:No. of H- bond = 2 * No. of AT base pairs + 3 * No. of GC base pairsHence, if 100 base pairs are present in a DNA structure with 30 AT base pairs and 70 GC base pairs the number of hydrogen bonds can be calculated as follows:No. of H-bond = ( 2 X 30 ) + (3 X 70 )= 270 H-bonds The DNA molecules that consist of more GC-rich regions are more stable due to more number of hydrogen bonds.Also, the splitting or breakage of DNA always begins from AT-rich regions as they are weakly bonded.
Why is Hydrogen Bonds Weak in DNA?
On an absolute scale the energy of a hydrogen bond ranges between 8 KJ/mol to 29 KJ/mol.Therefore, hydrogen bonds are much weaker as compared to covalent bonds in which the energy level ranges in hundreds.As hydrogen bonds are formed due to the force of attraction that exists between a hydrogen atom and an electronegative atom such as nitrogen, oxygen, etc. they are weak and their intensity decreases with bond length.The hydrogen bonding in the DNA molecule is ideal for its structure and functioning as it allows the optimum stability to the molecule also allows its unzipping during the replication process.
Conclusion
DNA is the hereditary material of most living organisms and helps in the transmission of biological information from one generation to another.DNA is a double-stranded structure formed of basic units called nucleotides that are made up of one deoxyribose sugar, one phosphate group, and one nitrogenous base.On the same strand, the nucleotides are bonded through phosphodiester linkages which are actually covalent bonds while on opposite strands nucleotides bond through hydrogen bonds.The hydrogen bonds in DNA contain the exact amount of energy that can balance the structure and function of the molecule i.e. it provides enough stability to the structure but also allows the unzipping during the replication process.As per Chargaff’s rule adenine always bonds with thymine through a double bond and guanine bonds with cytosine through a triple bond.The number of hydrogen bonds in a molecule varies from one individual to other. However, it can be calculated using the following formula:No. of H- bond = 2 * No. of AT base pairs + 3 * No. of GC base pairs