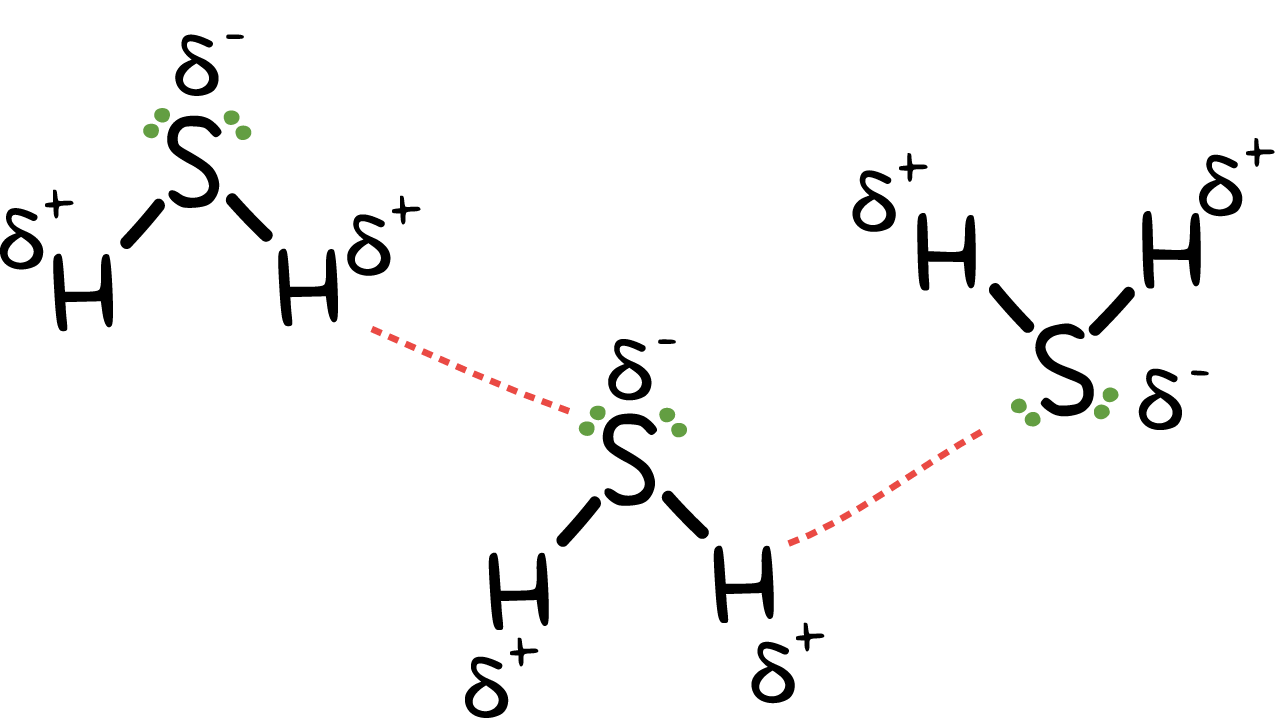
It is also produced as a product of the anaerobic digestion of organic matter by microorganisms. It burns to form sulfur dioxide and water, in the presence of oxygen. It is used in the production of sulfur, alkali metal sulfides, and thio organic compounds. It is also used in analytical chemistry for qualitative inorganic analysis of metal ions. It is also known as sewer gas or stink damp.Hello folks!!! We are here again with another interesting topic for you.In this article, we will talk about hydrogen sulfide and intermolecular forces. So, Let’s begin.So, What types of intermolecular forces are present in H2S?The dipole-dipole interactions and London dispersion forces are present as intermolecular forces between hydrogen sulfide molecules.The electronegativity of hydrogen is 2.2, while that of sulfur is 2.38. Due to this slight difference in electronegativity, the electrons shared in the formation of the bond shift slightly towards sulfur. As a result, positive and negative poles are created on the hydrogen and sulfur end of the molecules, respectively. Consequently, the dipole-dipole interaction develops between the molecules.The London dispersion forces are also present between the H2S molecules. However, these are the weakest of all the intermolecular forces and do not affect the properties of a molecule.
Why Does H2S Occur as a Gas While H2O is a Liquid?
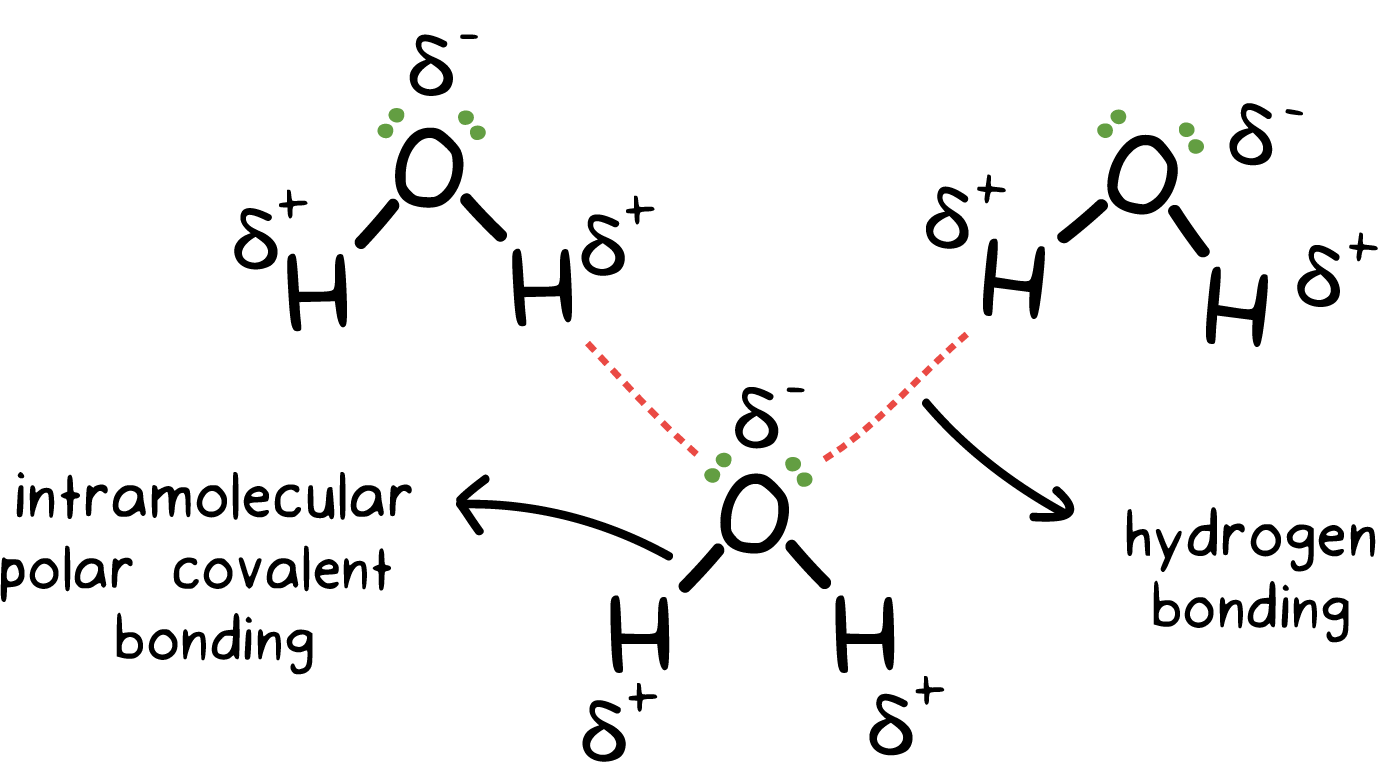
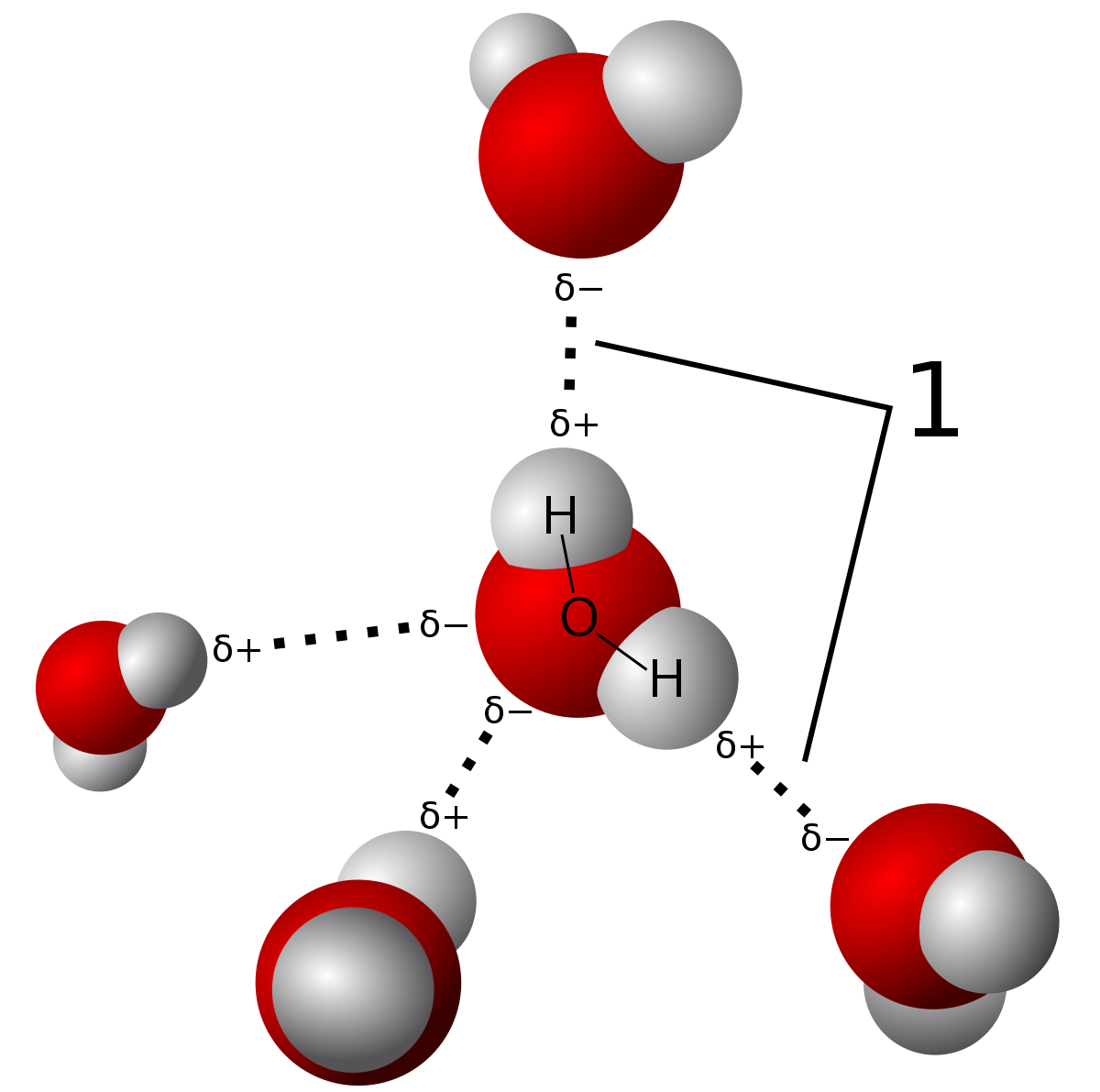
The intermolecular forces acting between the H2S molecules are dipole-dipole forces that are quite weak in comparison to hydrogen bonding which is present in between water molecules.The dipole-dipole interaction arises due to the electronegativity difference between the atoms combined to form a molecule.As the more electronegative atom pulls the shared electron pair slightly towards itself, a slight negative charge develops on it.This also results in the development of a slight positive charge on the lesser electronegative atom and a polar covalent bond is formed.Further, owing to the separation of charges inside the molecule, opposite poles develop that cause the dipole-dipole interaction.However, as the electronegativity difference is quite low between hydrogen (2.2) and sulfur (2.38) atoms, the forces developed are not as strong to keep the molecules held with each other.Thus, the molecules are more or less free to move away from each other and hence, H2S occurs as a gas.The other types of forces known to occur between the hydrogen sulfide molecules are the London dispersion forces.These are very weak in nature and occur in almost all the molecules, therefore, do not play many roles in defining the state of occurrence of a molecule.On the other hand, the H2O molecules are linked through hydrogen bonding, which is a strong intermolecular force.There is a vast difference in the electronegativity of hydrogen (2.2) and oxygen (3.44) atoms. This results in the formation of defined poles on the two ends of the molecules.These poles attract the opposite poles of the other molecules towards themselves i.e., the partially positive hydrogen atoms of one molecule attract the partial negative atom of oxygen. This results in the formation of strong bonds between different molecules.Hence, the H2O molecules remain closer to each other, and thus, water exists in the liquid state.Check out the below articles:Is H2O Polar or NonpolarIs H2O Ionic or CovalentH2O Lewis Structure, Geometry, Hybridization
Types of Intermolecular Forces
The forces of attraction that exist between different molecules of a compound when they are placed close to each other are known as intermolecular forces.Mostly, these are the electrostatic interactions that also play a role in determining the state in which that compound will occur. For example, dipole-dipole interaction, hydrogen bonding, etc.They are different from the intra-molecular forces between two or more atoms inside the same molecule.Intra-molecular forces may sometimes be responsible for determining the intermolecular forces that will arise between the molecules.For example, an ionic or polar covalent bond usually results in the dipole-dipole interaction.Different types of intermolecular forces are described below:• Ion-ion forces: The electrostatic forces of attraction that occur between the two or more ions. The oppositely charged ions are attracted to each other and result in the development of ion-ion forces.• Ion-dipole moment: This type of intermolecular force exists between a polar and an ionic molecule.In this case, a positive ion is attracted to the slight negative end of a polar molecule. Similarly, a negative ion is attracted to the slightly positive end of the polar molecule.A classic example of these forces is salt dissolved in water. The salt forms ions that bond with the polar molecules of water.• Dipole-dipole interaction: As also discussed in the case of H2S, these are the forces developed between the polar molecules. The polarity results from the difference in the electronegativity of the combining atoms, as between hydrogen and sulfur.These forces develop when in a polar covalent bond, a slight positive and slight negative charge develops on the two ends of a molecule. The molecules attract and, in turn, get attracted towards the opposite poles of other molecules.HCl compound is also one of the examples having dipole forces among its molecules. Check out the article on HCl Intermolecular Forces.• Hydrogen Bonding: These are the stronger version of dipole-dipole interactions. These forces also exist between the polar molecules of a compound.However, the only condition is that the electronegativity difference between the atoms has to be high. Due to this, hydrogen bonding only exists between the molecules in which hydrogen is bonded with oxygen, nitrogen, or fluorine.• London dispersion forces: These are the weakest intermolecular forces. These occur amongst all the molecules but are not strong enough to hold them close. These are also referred to as induced dipole-induced dipole forces.These forces usually appear owing to the development of temporary poles inside a molecule.The poles may develop due to the temporary induced charge on the molecule, which interacts with the similar temporary charge on the other molecule. Hence, the name induced dipole-induced dipole.The decreasing order of strength for the different types of intermolecular forces is as follows.Ion – Ion > Ion – Dipole > Hydrogen Bonding > Dipole-Dipole > Dipole-Induced Dipole > Induced Dipole-Induced Dipole forces
Why Does Hydrogen Bonding not occur in H2S?
Hydrogen bonding is the special intermolecular force that only exists between the molecules in which the hydrogen atoms are bonded to a highly electronegative atom such as fluorine, oxygen, or nitrogen.Due to the large difference in the electronegativity of these atoms, the opposite poles develop on the molecule.The more electronegative atom acquires a slightly negative charge while the less electronegative atom acquires a positive charge, just as in the case of dipole-dipole interaction.The electronegativity difference is quite high, so the poles so developed almost behave as an ion. They exhibit strong attraction forces towards the opposite pole of other molecules.Hence, strong electrostatic forces exist between the molecules of these compounds due to which they remain close to each other. This is responsible for the liquid state and the high melting point of water, hydrogen fluoride, and ammonia.However, in the case of H2S, the electronegativity difference between hydrogen and sulfur atom is not enough for the formation of strong and stable poles. Thus, hydrogen sulfide does not exhibit hydrogen bonding.
Does H2S have a Dipole Moment?
In the hydrogen sulfide molecule, the hydrogen and sulfur atoms are bonded through a polar covalent bond. This is due to the electronegativity difference between these two atoms that the two opposite poles develop inside the molecule.The dipole moment of a molecule is the measure of its polarity. Although the electronegativity difference between the hydrogen (2.2) and sulfur (2.38) atom is still low, H2S is a polar molecule.The value of dipole moment for H2S is 0.97 D. Question: Is H2S ionic or covalent?Answer: A covalent bond is formed by the sharing of electrons participating in forming a bond. As the valence electrons of sulfur and oxygen taking part in the formation of bonds are shared between these atoms, H2S is a covalent compound.Question: Is H2S a binary compound?Answer: Binary compounds are formed by the combination of two elements. Each H2S molecule is formed with hydrogen and sulfur atoms; H2S is a binary compound.Question: What kind of acid is H2S?Answer: H2S is a weak acid as it has a low value of acid dissociation constant. It occurs as gas and has a pKa value between 6 and 7. Related TopicsIs H2S Ionic or CovalentH2S Lewis Structure, Geometry, HybridizationIs H2S Polar or NonpolarIs HCl Polar or NonpolarHCl Lewis Structure, Geometry, HybridizationHF Lewis Structure, Geometry, Hybridization
Properties of H2S
A few important properties of hydrogen sulfide are given below:• The molecular mass of H2S is 34.08 gm/mol.• It is a colorless, pungent-smelling gas, with an odor like that of rotten eggs.• The density of H2S is 1.363 gm/dm3.• The melting and boiling points of Hydrogen sulfide are −82 °C and -60 °C, respectively.• The molecule is bent in shape with a dipole moment of 0.97D.
Conclusion
The intermolecular forces of attraction between H2S molecules are dipole-dipole and London dispersion forces.The dipole-dipole interaction results from the electronegativity difference between the combining atoms.The decreasing order of strength of different intermolecular forces is:Ion – Ion > Ion – Dipole > Hydrogen Bonding > Dipole-Dipole > Dipole-Induced Dipole > Induced Dipole-Induced Dipole forces