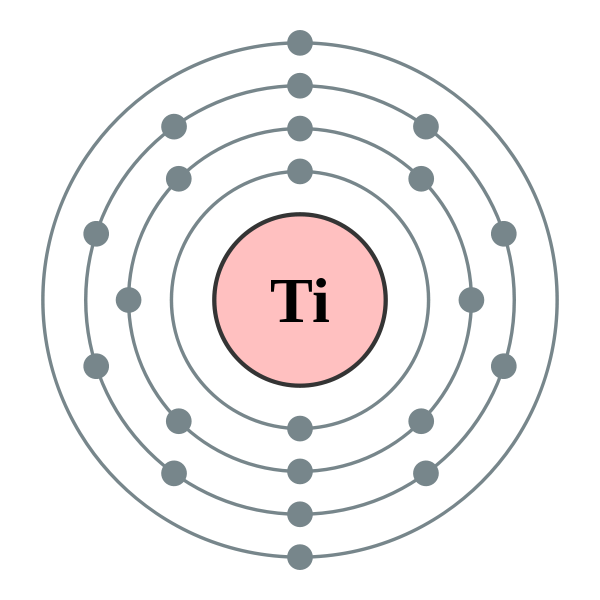
Since titanium possesses two most important properties – high strength-to-density ratio and corrosion resistance, it is widely used in aircrafts, chemicals and petrochemicals, orthopedic implants, sporting goods, military goods, mobile phones, automotive etc.In this article, we will discuss some of the most searched questions related to Titanium like-• Electrical conductivity of titanium• Resistivity of titanium• Do titanium implants conduct electricity?• Properties of titaniumSo, does titanium conduct electricity? Titanium is a poor conductor of electricity. The atom has its outermost orbital 4s2 completely filled thereby outermost electrons are unavailable for carrying current and electrons in the down orbital 3d2 are available but are more under the hold of the nucleus making it a poor conductor of electricity.Electrical conductivity is defined as the ability of a substance to permit the flow of electrons. In general, copper is used as the standard for determining the electrical conductivity of any material.So as compared to the electrical conductivity of copper, titanium exhibits only 3.1% of it. From this it follows that titanium conducts electricity; however, it would not be preferred like Aluminum, Copper, and silver where good conductivity is a prime factor.
Why is Titanium a Poor Conductor of Electricity?
There is a relationship between the electrical conductivity of an element and the arrangement of electrons in its outer atomic orbitals. Let’s understand this by comparing Aluminium (a good conductor of electricity) and Titanium.The atomic number of Aluminium is 13 and its corresponding electronic configuration is [Ne] 3s2 4p1.Whereas the atomic number of Titanium is 22 and its corresponding electronic configuration is [Ar] 3d² 4s².Now, it can be noticed that the outermost orbital in Aluminium ie, 4p is partially filled. It has one free electron that can be easily shared among adjacent atoms or molecules in the crystal and transfer electric charge throughout the metal.However, in the case of Titanium, the outermost orbital ie, 4s is completely filled and the next one down ie, 3d is partially filled. It means that electrons are available to share but not in the outermost orbital like Aluminium.Electrons present in the inner orbitals are a bit more closely linked with the nucleus so it is not easy for an atom to release them easily. As a result, when compared to other metals like Aluminium, titanium is a poor conductor of electricity.However, it is still much better at conducting electricity than an insulating material.
Do Titanium Implants Conduct Electricity?
Since the mid-1960s, titanium implants have been surgically implanted into patients’ jawbone to mimic the functions of a natural tooth root. These biocompatible dental implants are incredibly durable and lightweight.However, here the question is – do they conduct electricity?As titanium is a metal so it is natural to assume that it is electrically conductive. It is even hypothesized that the electro-conductive property of titanium implants forms a closed electrochemical circuit that results in the corrosion of titanium implants.In the case of titanium, it is not easy to delocalize its electrons to carry electric charge throughout the metal because they are not available in its outermost orbital. So, it conducts electricity but its electrical conductivity is quite low as compared to other metals.In short, the electro-conductive property of titanium may contribute to the corrosion of implants in the acidic system over the years but titanium is considered to be safe for orthopedic implant applications.
What is the Electrical Conductivity of Titanium?
Electrical conductivity is the fundamental property of a material to allow the flow of electric current through it.Materials with good electrical conductivity easily transfer the electric current whereas metals with poor or low electrical conductivity resist the flow of electric current. It is denoted by the Greek letter σ (sigma).Besides the nature of the material (metal or non-metal), factors like temperature, electromagnetic field, and frequency of electric current affect the electrical conductivity of the material.
Electro-magnetic field:
Electro-magnetic fields are present everywhere so, sometimes their presence with strong intensities interrupt the flow of current through a material.
Frequency of electric current:
Generally, an increase in the frequency of electric current increases conductivity but when increased above a certain higher limit (above 3 GHz), it causes a reduction in the electrical conductivity of the material.
Temperature:
In case of metals (having a large number of free electrons), the temperature is inversely proportional to the conductivity.An increase in temperature leads to the thermal excitation of particles. As a result, the increased movement hinders the path of current flow and decreases the conductivity.The table represents the electrical conductivity of materials at 200C
Metals that don’t Conduct Electricity
In general, metals have a tendency to lose electrons. These free electrons in the matrix start moving from the negative end to the positive end when the potential difference is applied across the terminals of the metal.This gives rise to the flow of current through the metal.However, there are some metals like Manganese (σ (S/m) = 0.69× 106) and Titanium (σ (S/m) = 2.38×106) which show low electrical conductivity because of absence of free electrons in valence orbitals.Electronic configuration of Manganese and Titanium are as follows-Mn – [Ar] 3d5 4s²Ti – [Ar] 3d2 4s²Both of them have completely filled outermost orbitals and partially filled next one inner orbitals.So, unlike good conductors copper and silver, they do not release their valence shell electrons to carry electric flow.Similarly, the electrons from partially filled inner orbital (d) are also not readily available as they are held more firmly by the nucleus.
Resistivity of Titanium
Resistivity is defined as the ability of the material how strongly it resists the flow of current through it. It is commonly denoted by the Greek letter ρ (rho). Factors affecting the resistivity of the material are as follows –
Temperature:
The resistivity of materials changes with the variation in the temperature. In case of metals, the resistivity increases with the increase of temperature whereas, it decreases in semiconductors and insulators.
Alloying:
Alloying increases the resistivity of the metal considerably. For example, silver has the lowest resistivity among all metals but adding the impurity of silver in copper can increase the resistivity of the latter.
Mechanical Stressing:
Do you know the hard-drawn copper has more resistivity than annealed copper?It is because mechanical stressing develops localized strains that disturb the movement of free electrons and thus increase resistivity. On contrary, annealing relieves the mechanical stressing and decreases resistivity.The table represents the resistivity of materials at 200C
Properties of Titanium
Physical Properties of Titanium
Crystal structure: Hexagonal close-packedMelting point: 1941 K High melting point of Titanium makes it useful as refractory material Boiling point: 3560 KDensity: 4.506 g/cm3Hardness: Highest strength-to-weight ratio of all metalsTitanium is a unique metal with low density and high strength. It is as strong as steel but 45% lighter than steel. This combination makes it an ideal candidate for alloying.Magnetic ordering: ParamagneticParamagnetic substances have small and positive susceptibility thus, they are slightly attracted by a magnetic fieldElectrical conductivity: 2.38 × 106 S/mThermal conductivity: 21.9 W/(m⋅K)As compared to other metals, titanium has fairly low electrical and thermal conductivity; however, still do better than insulators.
Chemical Properties of Titanium:
Titanium belongs to d block and is classified as a transition metal. So, it can use electrons from more than one of its electron shells (s and d) to make the bond with other elements.It is a reactive metal. Its surface immediately gets oxidized upon exposure to air and forms a passive layer of Titanium dioxide. When it first forms, it is a thin layer, but it continues to grow with time and prevents the metal from corrosion.Oxidation States: 4, 3, 2Specific Heat: 520 J/kg*KElectronegativity: 1.54 (Pauling scale)Heat of Fusion: 18.7 kJ/molHeat of Vaporization: 425 kJ/molRelated TopicsDoes Carbon Fiber Conduct ElectricityDoes Brass Conduct ElectricityDoes Ice Conduct ElectricityDoes Stainless Steel Conduct ElectricityDoes Rubber Conduct ElectricityDoes Dielectric Grease Conduct ElectricityDoes Leather Conduct ElectricityDoes Aluminum Conduct ElectricityDoes Copper Conduct ElectricityDoes Gold Conduct Electricity
Conclusion
Titanium is often regarded as the “metal of the future” because of its advantageous properties like high strength, low density, and corrosion resistance. Moreover, its alloys add greater malleability, ductility, and flexibility. It is a transition metal that belongs to Group 4 in the periodic table. It has two electrons in the d-orbital and two electrons in the s-orbital which makes its outermost orbital completely filled and d-orbital partially filled. As a result, it conducts electricity with low conductivity.The electrical conductivity and Resistivity of Titanium are – 2.38 × 106 S/m and 4.20 ×10-7 Ω⋅m respectively.