It also forms an essential trace element for most living organisms. Copper finds its use in different industries like construction, electronics, etc., and also in decorative items.In this article, we will check out whether copper also rusts like iron.So, does Copper rust? No, Copper does not rust. However, it undergoes oxidization or corrosion. Unlike rusting copper forms a bluish-green color layer on its surface, known as patina after corrosion, resulting in tarnishing its lustrous surface. As Copper is susceptible to losing electrons, it undergoes oxidation when exposed to air and humid environment and gets corroded over time.Actually, rusting is a type of oxidation reaction that occurs particularly in iron and its metal alloys when they are exposed to air and water like in a humid environment.It results in the formation of a reddish or yellowish-brown colored coating on the surface which is known as rust. So, technically, rusting is the property limited to iron and related compounds.Copper does not undergo rusting. However, it does get corroded which is another type of oxidation reaction. It is an electrochemical process that occurs when copper comes in contact with another electronegative element that has a strong urge to gain an electron, such as oxygen, or an electrolyte solution, such as water.Corrosion of copper is different from rusting of iron as the rust formed on the surface of the iron objects degrades and weakens the metal and also exposes the inner surfaces for further decay by rusting as the upper layer flakes away with time.On the other hand, the patina layer formed on the surface of copper works as a protective covering by sticking to the object and preventing further damage to the metal. Also, rusting makes the surface of metal appear unsightly, however, the patina layer gives the object an aesthetic appearance.
How long does it take for Copper to corrode?
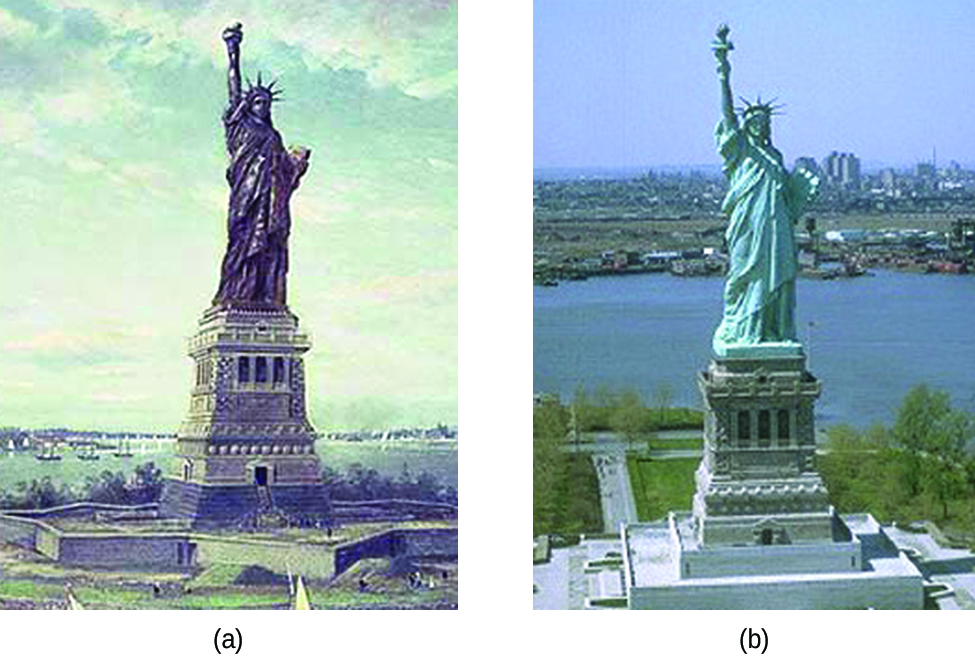
Copper is one of the few metals that occur in almost pure usable form in nature. The effect of corrosion is almost negligible on copper in the natural environment.However, the effects do get enhanced under extreme conditions such as saltwater, sulfur, ammonia, oxidizing acids, etc. Under favorable conditions, it takes as long as 20 years for copper to corrode.Actually, copper is one of the least reactive metals. It is placed fourth lowest in the reactivity series after gold, silver, and platinum which are noble metals.In fact, it is the low reactivity of copper due to which its cupronickel alloy (90% copper and 10 % nickel) is suitable for making various parts of water boats and ships.Have you seen the bluish-green coat on old pennies, it is due to the tarnishing of copper. The water pipes made of copper undergo erosion carrion in the long run due to the water’s turbulent flow.Also, did you know that the statue of liberty is actually made of copper? Now you know the reason for its bluish-green color.This layer is now almost as thick as its original copper layer and prevents further oxidation of inner copper atoms, helping preserve this heritage.
Why Does Copper Tarnish?
Copper has the electronic configuration [Ar] 3d10 4s1.This reveals that the copper atom is one electron away from attaining stability. It is due to this extra electron that copper reacts with electronegative atoms such as oxygen.This reaction causes copper to oxidize due to which it forms copper oxide and in some time also causes the formation of the bluish-green colored patina coating over the surface of copper articles.The patina layer serves as the protective coating for copper by covering the inner copper atoms and saving them from further oxidation.This reaction further gains pace under humid conditions when the moisture present in the air serves as a catalyst for the reaction. That is the reason why it is always advised to dry the copper articles or jewelry every time they come in contact with water.There are other factors that are responsible for the tarnishing of copper such as:• Heat: The corrosion process picks up speed under high temperatures which are readily available these days due to climate change.• Acidic condition: The lower pH also acts as a reaction accelerator. The acid rain and the sulfur gases present in the atmosphere due to high pollution levels also make the copper articles susceptible to corrosion.• Cleaning chemicals: They affect the outer layer of copper making it susceptible to corrosion.
Why does Copper turn green, blue, or black?
The changing colors encountered during the tarnishing of copper are due to the formation of different oxides of copper, as it comes in contact with oxygen in the presence of moisture.The different oxides are formed owing to the availability of copper atoms from the article and oxygen atoms from the atmosphere.The red color that appears on the copper surface is due to the formation of copper (I) oxide. This slowly changes to dark brown or blackish appearance which is actually copper (II) oxide.At later stages, the coating turns bluish-green, which is also known as patina, due to the formation of more complex oxides of copper.
Does Copper Corrode in Water?
Yes. Copper corrodes in water with or without the presence of dissolved oxygen.The corrosion that occurs in the absence of dissolved oxygen is known as anoxic corrosion. In this type of corrosion, hydrogen gas is released as a result of the reaction between copper atoms and water molecules.Theoretically, the corrosion occurs underwater when the pressure of hydrogen is and the concentration of copper ions both are low.The reaction occurs as follows:Cu + H2O —–> CuOH + ½ H2However, being pressure-dependent the reaction reverses as the pressure of hydrogen increases and again results in the formation of copper ions.
The Chemistry behind Corrosion of Copper
As discussed in the earlier section there are several stages of corrosion in copper that result in the formation of different products. The conditions for the formation of these products also vary.In this section let us try to understand the chemistry behind the corrosion of copper.When oxygen atoms first come in contact with copper atoms they result in the formation of red-colored copper(I) oxide as per the following reaction:4Cu (s) + O2 (g) —–> 2Cu2O (s)As the reaction further advances, the copper (I) oxide reacts with more oxygen atoms resulting in the formation of black-colored copper (II) oxide.2Cu2O (s) + O2 (g) —-> 4CuO(s)The further reactions occur due to the presence of various impurities present in the air. The different reaction equations are given below:2CuO (s) + CO2 (g) + H2O (l) —-> Cu2CO3(OH)2 (s) 3CuO (s) + 2CO2 (g) + H2O (l) —-> Cu2(CO3)2(OH)2 (s) 4CuO (s) + SO3 (g) + 3H2O (l) —–> Cu4SO4(OH)6 (s)These complex copper oxides are responsible for the bluish-green patina layer that covers the copper structures and also protects them from further corrosion.
Best ways to Clean Copper
There are varieties of cleaners available in the market for cleaning copper products. Also, as copper has been used by humans since prehistoric ages many household solutions are also available.One thing common amongst them all is they are mildly acidic due to which they form copper salts which can be washed away with water giving your product a new-like appearance.Below are a few of the recognized solutions for cleaning copper articles.• Market-bought copper cleaners: A number of products are available in the market in the form of pastes or cream that clean the copper articles without damaging their surface.• Worcestershire sauce: This acidic sauce can clean the copper’s tarnished surface, giving it a shiny look.• Tamarind paste: It also has acidic properties due to which it can work as a cleaner for copper articles.• Lemon juice plus baking soda: Lemon is acidic in nature and baking soda helps in scrubbing away of the tarnished surface thus cleaning the copper articles professionally.• White vinegar and salt: 1½ teaspoon of salt mixed in a cup of white vinegar serves as an excellent cleaner for copper.
How to prevent corrosion of Copper
Copper oxidizes naturally when exposed to humid conditions resulting in the tarnishing of its surface. However, there are certain ways to protect copper articles from corrosion.A few of them are listed below:• Greasing: This forms a protective layer around the outer surface minimizing exposure to humid conditions. Any household grease such as Vaseline may help prevent the corrosion of copper.• Paint Sealers: The sealer keep the air away from the copper articles. This can be an effective solution in the case of display objects that are not handled regularly.• Cleaning: Most basic but the most effective solution to keep corrosion at bay. Cleaning the copper articles regularly and keeping them dry is a great way to prevent tarnishing.
Conclusion
Copper does not rust like iron. However, it undergoes another type of oxidation reaction called corrosion.The corrosion of copper is different from rusting as the rusted iron objects become weak and fragile. The rust layer keeps flaking away, exposing the inner layers and further degrading the object. Corrosion of copper on the other hand forms a protective covering on the surface that prevents the inner atoms from further damage.Copper is one of the least reactive metals that takes a long time to corrode.The main reason copper reacts with oxygen atoms is the presence of one 4s electron, which can be easily removed by an electronegative atom making the copper atom stable.The different color changes during the corrosion of copper are due to the formation of different copper oxides.


:max_bytes(150000):strip_icc()/copper-and-brass-tarnish-remover-1387940_03_WhatYouNeed-ee6b7d1ccce246c2ab6ee4b2de1c9b9d.jpg)