The main uses of ammonia are for making fertilizer for agriculture or as an intermediate in chemical industries.Direct uses of ammonia are as a working fluid in refrigeration cycles and household cleaning solutions. However, only about 4% of ammonia is used in direct applications.However, experts expect ammonia consumption as a fuel due to environmental concerns, mainly to reduce CO2 emissions.Yes, the uses of ammonia are many. In this article, we will explore the main applications of this essential chemical. But first, let’s learn about its properties.Ammonia is found in nature, being produced by many organisms. Ammonia is a natural byproduct of biological and chemical reactions, including the decomposition of organic matter, including plants, animals, and animal wastes.However, it is caustic and hazardous in its concentrated form (pure or as a water solution).It is made in huge quantities (estimated at 144 million metric tons in 2020), mainly used in the production of fertilizers. Also, it is present in many commercial cleaning products and the preparation of pharmaceuticals.People quickly detect ammonia by its strong, pungent odor at concentrations well below the risk level.The average odor threshold is 5 ppm (parts-per-million), well below any danger or damage. However, exposure to high concentrations of gaseous ammonia can result in lung damage and death.This table shows that ammonia has a low melting and boiling points. Thus, this compound is a gas under normal conditions.Solubility in water, 50 °C, w/w18%Pure ammonia is shipped in special containers under high pressure or low temperatures. Ammonia is also transported as concentrated solutions in water.The ammonia molecule is a Lewis base (it can donate its free electron pair). Ammonia water solutions are basic due to the following reaction: NH3 + H2O <====> NH4+ + OH–In this reaction, ammonia also behaves like a Bronsted-Lowry base because it accepts a proton (H+) from water.
Production and Distribution of Ammonia (NH3)
The main route for the production of ammonia is the following reaction:N2 + 3H2 → 2NH3With nitrogen separated from the air and hydrogen obtained from natural gas. This synthesis is called the Haber-Bosch process. Fritz Haber discovered the reaction in 1909, and Carl Bosch developed the industrial-scale process (applied to the production of fertilizers) in 1914.This reaction proceeds under conditions of high temperature and pressure in the presence of a catalyst.
How is Ammonia (NH3) shipped and sold?
Anhydrous ammonia is supplied under pressure in steel tanks as a liquid. The commercial aqueous solution contains 25% ammonia, transported in steel tanks or plastic containers.However, the concentration of ammonia solution for household use is only 5-10%.
Present Applications
Cleansing agent
A diluted solution of ammonia in water (5-10%), known as “household ammonia,” is useful for cleaning many surfaces and cutting grease.The basic ammonia solution will react with the free fatty acids in the grease or vegetable oils, making a “soap” that will dislodge the dirt.One advantage is that ammonia solutions leave a streak-free surface because both ammonia and water evaporate.Thus, it is commonly used to clean glass, stainless steel, and porcelain surfaces, for example, windows, ovens, tubs, sinks, toilets, countertops, and tiles.The “household ammonia” products irritate the eyes, mucous membranes (respiratory and digestive tracts), and the skin. In the case of eye and skin contact, wash with plenty of water.People should take special care to avoid mixing ammonia solutions with cleaners containing chlorine or hypochlorite (bleach) because the combination produces volatile, very toxic compounds known as chloramines.
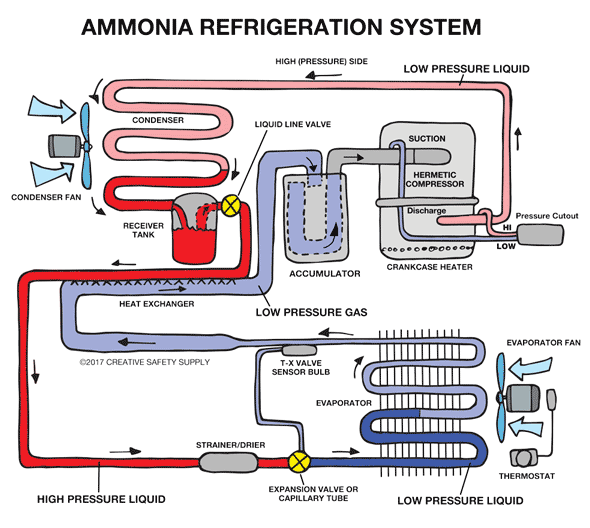
Industrial Refrigeration
Ammonia is the most commonly used refrigerant worldwide for large commercial applications because it is a very effective fluid for refrigeration.In fact, ammonia is a much more efficient cooling fluid than those found in home refrigerators.The use of ammonia in home refrigerators was phased out a long time ago because it is poisonous and dangerous in case of a leak. However, ammonia is easily detected by its smell at very low concentrations.
Fertilizers
The element nitrogen is one of the essential nutrients for plants.However, plants cannot “fix” nitrogen from the air, so fertilizers add nitrogen to the soil to make plants grow quicker, larger, healthier, and more nutritious.Also, fertilizers increase harvest yields, reducing the cost of production. Therefore, 80% of the ammonia produced is dedicated to the synthesis of fertilizers.
Ammonia as fertilizer
Ammonia is useful as a fertilizer for home gardens. A shower of a ¼-cup of household ammonia diluted in 1 gallon (3.7 liters) water will give a boost in nitrogen to plants and vegetables.
Ammonia for slug control
Slugs are pests. Gardening sites propose to use a diluted solution (1 part household ammonia to 9 parts of water) to spray in the soil or over the plants. However, some recommend spraying the slugs directly.
Ammonia as the precursor to fertilizers
Ammonia is used to prepare fertilizers that improve crop yields and feed the world.The fertilizers ammonium nitrate and urea are made in industrial amounts by the following reactions:First, the oxidation of ammonia gives nitric acid:NH3 + 2O2 → HNO3 +H2OThis reaction occurs at high temperatures in the presence of a catalyst.Then, nitric acid reacts with ammonia to make ammonium nitrate:HNO3 + NH3 → NH4NO3The reaction of ammonia with carbon dioxide gives urea, another important fertilizer:2NH3 + CO2 → (NH2)2C=O + H2O The following diagram resumes the processes:Ammonium nitrate and urea can be mixed with water to form a UAN (urea ammonium nitrate) solution.
Ammonia as a precursor to explosives
Ammonium nitrate is used to prepare explosives.By itself, ammonium nitrate is not a strong explosive. But it belongs to a class of compounds known as oxidizers, that react with combustible material, like fuel oil and aluminum powder.
The precursor to other nitrogenous compounds:
Many nitrogen-containing compounds of industrial importance are made starting with ammonia:
Hydrazine (NH2-NH2):
Hydrazine finds many applications, including the preparation of pharmaceuticals, explosives, polymers, and organic chemicals.One method for hydrazine production is the Peroxide Process, which uses hydrogen peroxide, H2O2. The net reaction is:2NH3 + H2O2 → NH2-NH2 + 2H2O
Hydroxylamine (NH2-OH):
Hydroxylamine is a reagent used in many organic and inorganic reactions, including the preparation of the polymer Nylon 6.The main route is via the Raschig process. The net reaction for the preparation of hydroxylamine is:2NH4NO2 + 4SO2 + 6H2O + 6NH3 → 4(NH4)2SO4 + 2NH2OH
Acrylonitrile
One of the main uses of acrylonitrile is as a monomer to prepare polyacrylonitrile (a homopolymer).Also, by reaction with other monomers, important copolymers are made, like styrene-acrylonitrile (SAN) and acrylonitrile butadiene (NBR) (a synthetic rubber).Production of acrylonitrile is done by catalytic ammoxidation of propylene, also known as the SOHIO process. The net reaction isCH3CH=CH2 + 3/2 O2 + NH3 ——> N≡CCH=CH2 + 3H2O
Ammonia as a clean fuel
The combustion of conventional fuels (gasoline, diesel, kerosene) produces enormous amounts of CO2, a greenhouse gas responsible for global warming.Ammonia does not contain carbon, and its complete combustion would not generate greenhouse gases or other contaminants:2NH3 + 3/2 O2 → N2 +3H2OThere are many investigations at present aimed at the utilization of ammonia as fuel. Also, there are plans in several countries to increase the production capacity of ammonia.The use of ammonia as a clean fuel has many potential advantages:• First, it is carbon-free as compared with gasoline, diesel, and kerosene (it will not produce CO2, a greenhouse gas).• It may replace gasoline, diesel, and kerosene in many applications• Leaks can be easily detected.• There is a lot of experience in the production and transportation of ammonia.• It has three hydrogen atoms in the molecule and may be used as a hydrogen carrier.• Its production, storage, transportation, and distribution are easier and less complicated than many other fuels.• It is cost-effective and economically feasible for applications.• All combustion systems, including engines and gas turbines, can be adapted to work with ammonia.• It can be a potential fuel solution for clean power generation in remote areas.However, producing ammonia with a non-contaminating process will be necessary for ammonia to be a proper clean fuel.Hydrogen from natural gas (as in the Haber-Bosch process) should be replaced by hydrogen made with renewable energies, like wind, solar, or biomass. Related posts you must readNH3 Lewis Structure, Geometry, Hybridization, and PolarityIs NH3 Polar or NonpolarDoes NH3 have hydrogen bondingIs NH3 Ionic or CovalentAmmonia is a chemical with many applications.Ammonia solutions find use mainly as cleaning agents.Ammonia gas is mainly used as a precursor to fertilizers and lesser to explosives.Many intermediates in the chemical industry are made from ammonia.There are plans to develop the use of ammonia as a fuel in the energy industry.